- PRODUCTS
- MODEL LIST
- APPLICATIONS
- SUPPORT
- SALES/SERVICE
- BLOG
- ABOUT
ISFET pH Sensors: Internal and External References
The functionality on this page was blocked because content blocking is enabled.
Please use another browser to access the form. We recommend Google Chrome. [Learn More]
Differences and knowing when to use pH Internal or pH External
Greg Ikeda
March 2019
Introduction
Like conventional glass electrode pH sensors, ISFET pH sensors require a reference electrode to make accurate measurements. Sea-Bird Scientific’s line of ISFET pH sensors utilize an Ag/AgCl reference electrode for this purpose. Due to the design of the ISFET sensor, it is the voltage on the reference (known as the “Reference Voltage”) that is used to calculate pH.
The Shallow SeaFET V2 and Shallow SeapHOx V2 pH sensors each utilize two separate Ag/AgCl reference electrodes. These instruments have an “internal reference electrode” which is semi-isolated from the surrounding environment, and an “external reference electrode” which is directly exposed to the surrounding seawater. The location and construction of each reference electrode has unique advantages and sources of error.
The resulting data from the SeaFET V2 and SeapHOx V2 contain two separate pH values referred to as “pH Internal” and “pH External”. While both should be close to one another after post-processing, deciding when to favor data from the internal reference or the external reference depends on the deployment environment and application. Selecting the correct source can have significant implications for data accuracy.
Reference Dependence on Chloride Ion Concentration
The basic principle of the ISFET sensor is as follows. In operation, the counter electrode attracts negative chloride ions to the ISFET to counteract the positive H+ ions present in the seawater. The Ag/AgCl electrode measures the chloride ion concentration needed to achieve this and thus the voltage on it changes with pH.

However, this means that to obtain an accurate pH, the effect of natural chloride ions must be corrected for. The internal and external electrodes do this correction differently.
Internal Reference
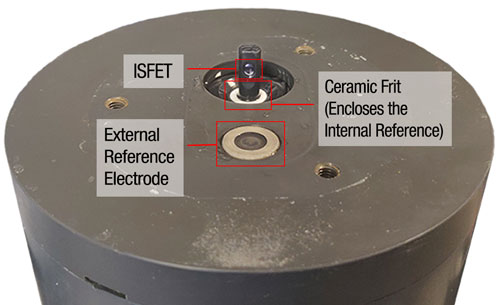
Housed within the SeaFET V2’s DuraFET, the internal reference electrode is immersed in a bath of saturated KCl gel that is physically separated from the environment by a ceramic frit. This frit allows hydrogen ions to pass through but also keeps the KCl gel from immediately mixing with the seawater. This arrangement exposes the Ag/AgCl internal electrode to a relatively constant chloride ion concentration and thus allows the internal reference to accurately measure pH regardless of the chloride concentration of the measured seawater.
However, this also means that measurement from the internal reference is dependent on a stable and known concentration of KCl in the gel electrolyte. Over time, the concentration of KCl will change as ions migrate out of the gel and into the surrounding water, resulting in sensor drift. It is due to this depletion of the gel electrolyte that shallow SeaFET V2 instruments must be serviced annually.
External Reference
The external reference electrode is also sensitive to chloride ion concentration, but unlike the internal reference, the external reference is not surrounded by a known and stable chloride concentration, since the number of chloride ions varies in seawater.
Fortunately, natural chloride ion concentration can be reliably estimated from seawater conductivity (salinity) as measured with a CTD. Thus, applying accurate salinity data to the pH External calculation will correct for the effect of natural chloride, allowing for an external reference measurement that is solely from pH.
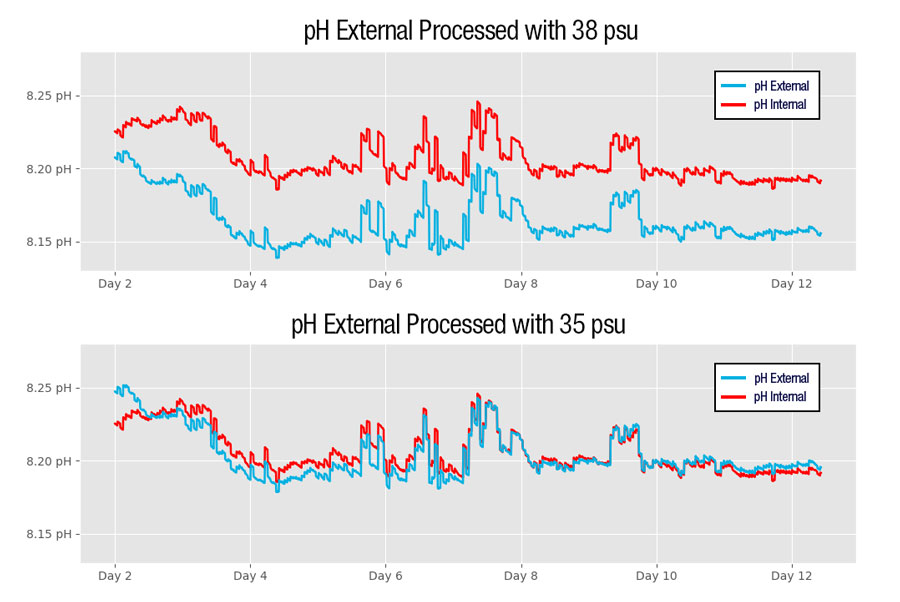
CTD Considerations
Salinity used for correcting pH External should be accurate to at least 0.01 psu and must be from the same water mass that is contacting the external reference electrode. In some cases, a CTD that is close to the pH sensor may be measuring a different salinity than the water contacting the external reference electrode. The SeapHOx V2 addresses this problem by providing accurate salinity from the 37-SMP-ODO CTD, as well as connecting the ISFET and external reference to the same flow path as the CTD’s conductivity cell to ensure that all sensors sample the same water mass.
When deploying a SeaFET V2 without a CTD, users must specify a static reference salinity used for processing pH external. If this reference salinity is reasonably close, pH External will be generally accurate. However, a static reference value fails to capture dynamic changes in salinity. Variations in salinity will appear as changes in pH, offsetting the accuracy of pH external whenever salinity varies from the reference value.
Field Data Example
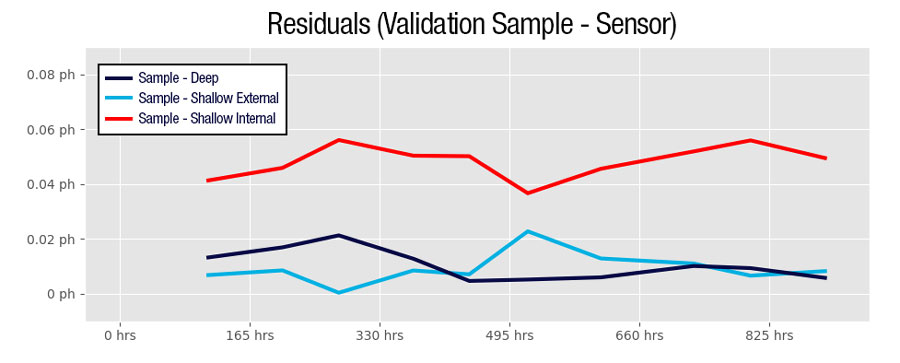
Data from one of Sea-Bird Scientific’s field experiments demonstrates the differences in pH Internal and pH External accuracy and stability over the course of a month-long deployment. In this experiment, A Shallow SeapHOx V2 and a Deep SeapHOx V2 (which does not have an internal reference electrode) were deployed next to one another in a dynamic estuarine environment. Ten validation samples verified by a spectrophotometer mark the true pH throughout the time-series.
Based on data from the validation samples, the Deep SeapHOx V2 was highly accurate (average sample – sensor residual = 0. 00948 pH) as was the Shallow SeapHOx V2’s pH External (average sample – sensor residual = 0. 00925 pH). The Shallow SeapHOx V2’s pH Internal started reasonably accurate, but appeared to drift over time (average sample – sensor residual = 0. 0483 pH). Recalibration of this unit showed that the DuraFET needed to be replaced after recovery, indicating that the internal KCl may have been close to depleted towards the end of the deployment.
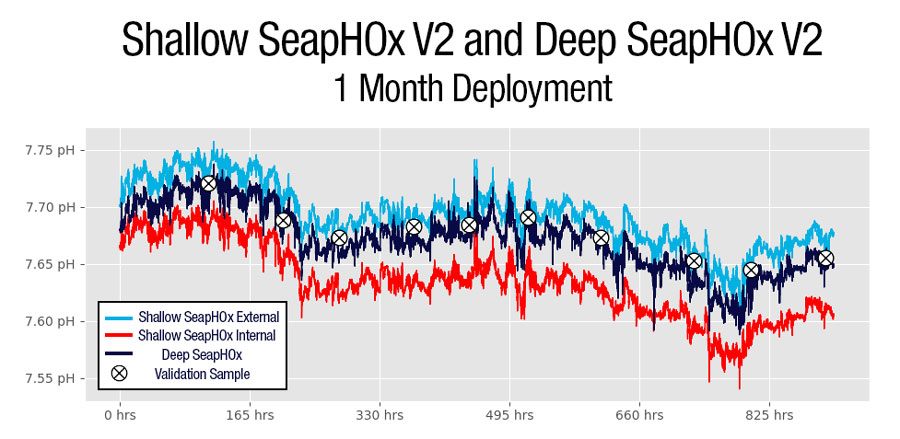
These data demonstrate that pH generated from the external reference electrode are more accurate when corrected with proper salinity; the overall difference from the validation samples is lower, and stability throughout the course of the deployment is more reliable. The internal reference was less accurate overall compared to the validation samples, and appears to have drifted throughout the course of the deployment (although much of this may have been hardware related).

When to Use pH Internal or pH External
To simplify, pH Internal is generally more accurate if salinity is unknown, especially in areas where salinity is changing rapidly—this is typical for deployments of a SeaFET V2 without an accompanying CTD. When accurate salinity data is available, pH External is generally more accurate. This is typically when deploying a SeapHOx V2, which combines the SeaFET V2 with a 37-SMP-ODO CTD. Ultimately, both pH values should be within 0.05 pH units of each other after post-processing, but processing pH External with the incorrect salinity can lead to significant error.
Follow some general rules when deciding which pH value to use:
- When using a SeapHOx V2: use pH External
- When using a SeaFET V2: use pH Internal
- When using a SeaFET V2 and a CTD is nearby: use pH External ONLY if you can be confident that the salinity measured by the CTD is close to the salinity contacting the SeaFET V2. If the sensors are deployed in an environment with dynamically changing salinity (such as an estuary), use pH Internal.

