- PRODUCTS
- MODEL LIST
- APPLICATIONS
- SUPPORT
- SALES/SERVICE
- BLOG
- ABOUT
Comparing ISFET and Glass Electrode pH Sensors
The functionality on this page was blocked because content blocking is enabled.
Please use another browser to access the form. We recommend Google Chrome. [Learn More]
Part 1: Stable Bath Test

An ocean mooring is like a small community: clusters of sensors huddle together, each measuring the ocean’s pulse in a different way, sometimes chatting with one another about what they’ve seen. As the world’s oceans become more acidic, it’s becoming increasingly clear that pH sensors should be a vital member of this community. However, the prevailing technology—glass electrode pH sensors—are notoriously unstable in seawater, necessitating frequent calibration and adding additional challenges to long-term deployments.
Enter the Sea-Bird Scientific SeapHOx: part pH sensor, part CTD. It measures pH with an Ion-Sensitive Field-Effect Transistor (ISFET) and corrects its data with temperature and salinity input from an SBE 37 CTD. Unlike glass electrodes, the SeapHOX’s ISFET and solid-state reference sensors are fit for long-term deployments, meant to retain accuracy for stretches of time without recalibration.

As part of ongoing efforts in pH sensor development, Sea-Bird Scientific conducted a basic comparison of the Shallow SeapHOx V2 to a HydroCAT-EP, a multiparameter CTD that uses a standard glass electrode pH sensor. Simultaneously deployed in a stable test bath for approximately 72 hours, the response from each sensor highlighted differences between each sensor technology.
This test provided a brief snapshot of sensor performance under salinity-stable conditions. Naturally, this is not an ideal test to characterize sensor differences— it fails to simulate rapidly changing field conditions, and was not long enough to capture drift rates of either pH sensor. Regardless, the data establishes a baseline for comparison to future field data.
This is Part 1 of a 2-part experiment. In Part 2, we will compare sensor performance from a long-term deployment in a dynamic estuary environment.

Left:HydroCAT_EP Glass Electrode pH sensor
Right: ISFET pH Sensor
The Bath Test
Both sensors were deployed simultaneously in a chilled bath of natural seawater for approximately 72 hours. At roughly 24 hour periods, we increased the temperature, which stimulated slight changes in pH. To determine which sensor was most accurate, we collected and analyzed 4 pH validation samples before each temperature change using standard pH indicator dye spectrophotometry. The SeapHOx equilibrated to the test bath for 72 hours prior to the experiment for optimal data quality.
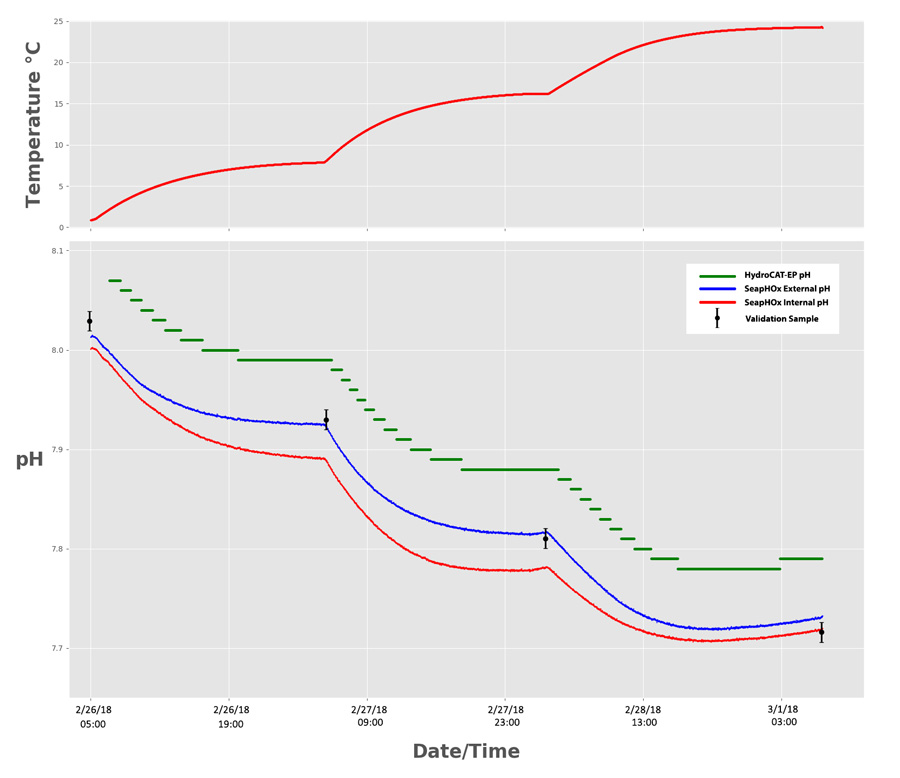
Accuracy
The Shallow SeapHOx has two pH outputs: “Internal pH” and “External pH”. While both originate from the ISFET as the primary sensor, each output comes from a different reference electrode. The Internal pH references an internal electrode submerged in saturated KCl solution, while the external pH references an Ag/AgCl electrode that is directly in contact with seawater. Each output has unique sources of error, but External pH is generally more accurate when salinity data is available for pH data corrections. Overall, both SeapHOx outputs were more accurate than the HydroCAT-EP, with an average difference from the validation samples of 0.0108 pH (External) and 0.0253 pH (Internal)—well within the rated accuracy spec of 0.05 pH.
The HydroCAT-EP also performed within its accuracy spec of 0.1 pH, retaining an average difference of 0.062 pH from the validation samples.
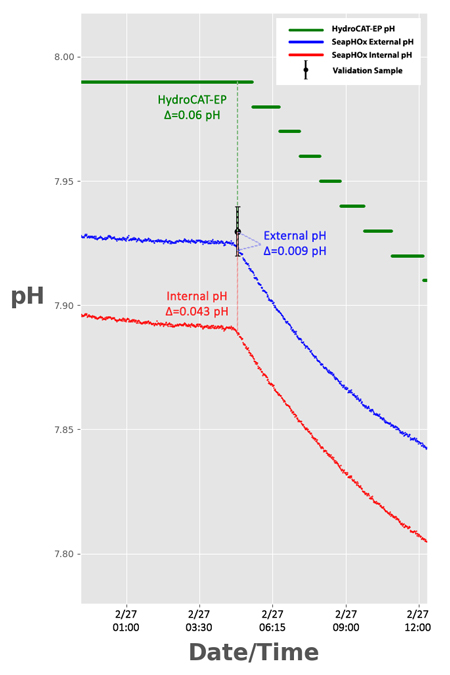
Fig 2. Enhanced view of the second validation sample, with the difference between pH from each sensor and the pH of the validation sample (Δ). All sensors are within their expected initial accuracy specification.
HydroCAT-EP pH Accuracy Spec
= 0.1 pH
SeapHOx Accuracy Spec
(Internal and External)
= 0.05 pH
Resolution
Superior resolution from the SeapHOx was immediately evident. The SeapHOx was easily able to track changes minor changes within 0.002 pH. The HydroCAT-EP’s pH sensor could only resolve changes of 0.01 pH, resulting in “stepwise” pH data.
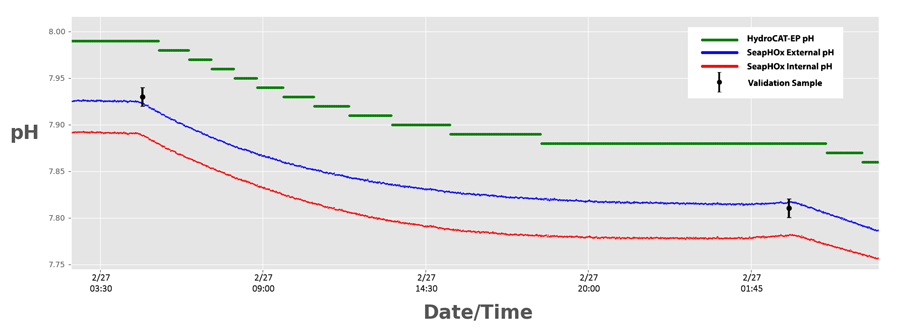
Better resolution has obvious advantages for tracking ocean acidification, especially in deep ocean environments where pH changes may be relatively small.
Next Steps
Overall both the ISFET and glass electrode pH sensors performed as expected, with the ISFET achieving approximately 3× better accuracy and 5× better resolution than the glass electrode in a controlled seawater bath. However, as mentioned earlier, a comparison in a stable bath is not a strong representation of how each sensor will perform in the field. Rather, it verifies that each instrument is meeting its expected accuracy and resolution, and the ISFET accuracy and resolution specs are superior to the glass bulb electrode on the HydroCAT-EP. Now, if either sensor performs poorly in the field, we can more easily investigate environmental factors or sensor issues that may be affecting data quality.
In Part 2 of this investigation we will compare how each sensor’s data quality holds up in a long-term field deployment, tracking drift and the instrument’s ability to remain stable in rapidly changing conditions.

